Dabiri, J.O., Colin, S.P., Costello, J.H. 2007.Morphological diversity of medusan lineages constrained by animal–fluid interactions. J. Exp. Biol. 210: 1868-1873.

Download the Paper (PDF File, 862 KB)
Abstract:
Cnidarian medusae, commonly known as jellyfish, represent the earliest known animal taxa to achieve locomotion using muscle power. Propulsion by medusae requires the force of bell contraction to generate forward thrust. However, thrust production is limited in medusae by the primitive structure of their epitheliomuscular cells. This paper demonstrates that constraints in available locomotor muscular force result in a trade-off between high-thrust swimming via jet propulsion and high-efficiency swimming via a combined jet-paddling propulsion. This trade-off is reflected in the morphological diversity of medusae, which exhibit a range of fineness ratios (i.e. the ratio between bell height and diameter) and small body size in the high-thrust regime, and low fineness ratios and large body size in the high-efficiency regime. A quantitative model of the animal-fluid interactions that dictate this trade-off is developed and validated by comparison with morphological data collected from 660 extant medusan species ranging in size from 300 micrometers to over 2 meters. These results demonstrate a biomechanical basis linking fluid dynamics and the evolution of medusan bell morphology. We believe these to be the organising principles for muscle-driven motility in Cnidaria.
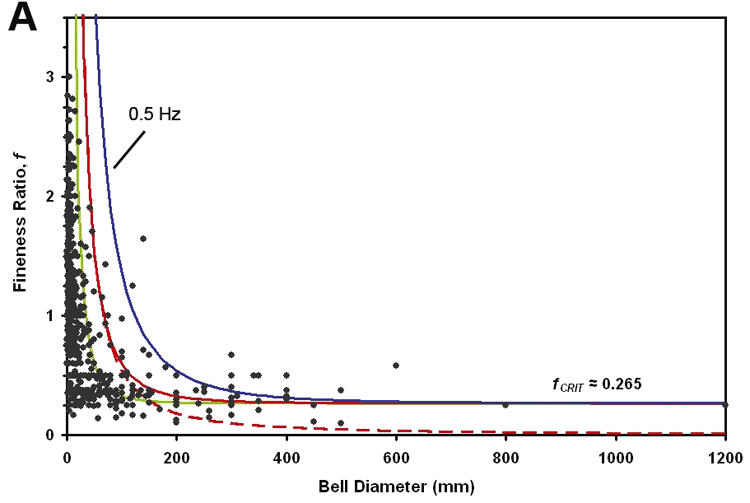
Fig. 1. Comparison of quantitative model of morphological diversity with data from 660 known species of medusae that supports the idea that medusan bell morphology is constrained by force requirements for jet propulsion. Black circles are morphological data; solid blue curve is the model result for a medusa that pulses one every 2 seconds (0.5 Hz swimming frequency model); solid red curve is the model result for a medusa that pulses once each second (1 Hz model); solid green curve is the result for a medusae that pulses two times each second (2 Hz model). The constraint on morphological diversity becomes more severe with increasing swimming frequency due to the increased flow accelerations and required locomotive forces. Dashed red curve is model prediction in the absence of stopping vortex formation that is characteristic of rowing propulsion (at 1 Hz). This demonstrates that if medusae are oblate (plate shaped) they are not constrained in size as a result of the lower force requirements of their rowing propulsion.
Costello, J.H., Colin, S. P., Dabiri, J.H. 2008. Constraints and consequences in medusan evolution. Invert. Biol. 127: 265-290.
(PDF File 1.57 MB)
Abstract:
Medusae were the earliest animals to evolve muscle-powered swimming in the seas. Although they have achieved diverse and prominent ecological roles throughout the world’s oceans, the limited cellular inheritance of the Medusozoa has constrained its ecology and evolution. We argue that the primitive organization of cnidarian muscle tissue limits force production and, hence, the mechanical alternatives for swimming bell function. We use a recently developed model comparing the potential force production with the hydrodynamic requirements of jet propulsion and conclude that jet production is possible only at relatively small bell diameters. In contrast, production of a more complex wake via what we term rowing propulsion permits much larger sizes but requires a different suite of morphological features. Analysis of morphometric data from all medusan taxa independently confirms size-dependent patterns of bell forms that correspond with model predictions. Further, morphospace analysis indicates that various lineages within the Medusozoa have proceeded along either of two evolutionary trajectories. The first alternative involved restriction of jet propelled medusan bell diameters to small dimensions. These medusae may either be solitary individuals (characteristic of Anthomedusae and Trachymedusae), or aggregates of small individual medusan units into larger colonial forms (characteristic of the nectophores of many members of the Siphonophorae). The second trajectory involved use of rowing propulsion (characteristic of Scyphozoa and some hydromedusan lineages such as the Leptomedusae and Narcomedusae) that allows much larger bell sizes. Convergence on either of the differing propulsive alternatives within the Medusozoa has emerged via parallel evolution among different medusan lineages. For example, rowing propulsion has evolved independently within the Scyphozoa, Leptomedusae and Narcomedusae. The variety of developmental pathways and morphological structures employed among medusan lineages to achieve either propulsive mode suggests that strong selective pressures underlie the polarized medusan morphospace. The distinctions between propulsive modes have important ecological ramifications because swimming and foraging are interdependent activities for medusae. Prey selection is therefore dependent upon foraging mode. Rowing swimmers are characteristically cruising predators that select different prey types than jet-propelled medusae that are predominantly ambush predators. These relationships indicate that the different biomechanical solutions to constraints on bell function have entailed ecological consequences that are evident in the prey selection patterns and trophic impacts of contemporary medusan lineages.
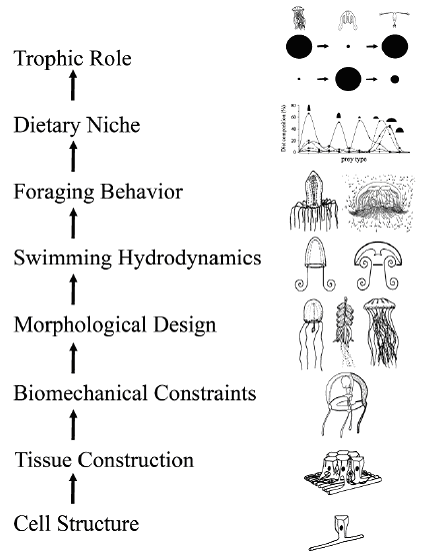
Fig. 2. Due to their relatively simple organization, the jellyfish represent an unusual opportunity to piece together causal relationships that connect cellular, tissue, morphological, biomechanical, behavioral, and trophic processes of an animal group. In this figure we depict these in a linear pattern summarizing the links which occur at each level. This schematic depiction is a simplification because many of the links between levels are bi-directional or interact in a more complex manner than the schematic synopsis. For example, prey availability certainly affects the success of particular dietary niches and foraging modes. In turn, the latter may affect natural selection on bell morphologies and propulsive modes. Yet despite its clear limitations, the synopsis provides a framework for understanding the integration of hierarchical levels of organization from cells to communities for one of the earliest planktonic animal groups.
We envision medusan evolution to be inextricably bound to this series of relationships with each successive level of biological organization, from cellular to ecosystem, emerging from constraining traits of preceding levels. In this way, the ‘primitive’ level of medusan organization provides a uniquely simple opportunity to assemble the interlocking parts of an evolutionary story. Our view begins at the cellular level but progress on the molecular and developmental levels may reveal even more fundamental patterns underlying medusan organization.
 Jellyfish evolutionary constraints
Jellyfish evolutionary constraints